2014
04 Dec 2014, Chemical Communications
Ru-Qiang Lu, Yi-Nyu Zhou, Xiao-Yun Yan, Ke Shi, Yu-Qing Zheng, Ming Luo, Xin-Chang Wang, Jian Pei*, Haiping Xia, Laura Zoppi, Kim K. Baldridge*,Jay S. Siegeld and Xiao-Yu Cao*
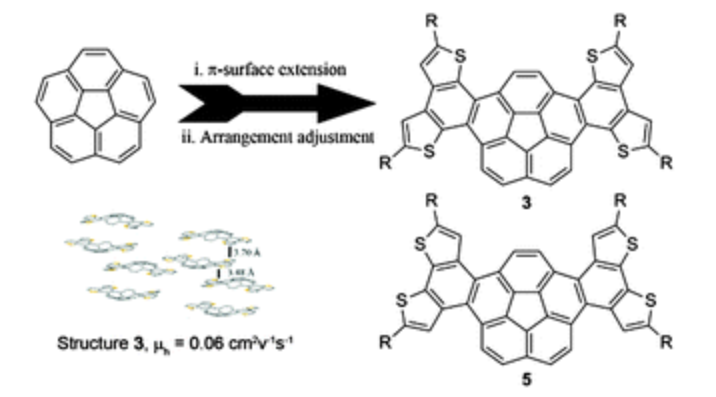
For the first time, electron-rich thiophene units were fused into the skeleton of corannulene to extend π-surfaces and tune arrangement in single crystals. Two isomeric butterfly-like thiophene-fused dibenzo[a,g]corannulenes (3 and 5) were synthesized. Isomer 3 showed p-type transport properties, with a hole mobility of 0.06 cm2 V−1 s−1.
04 Nov 2014, Polymer Chemistry
X. X. Tan, L. L. Yang, Z. H. Huang, Y. Yu, Z.Q. Wang, X. Zhang*
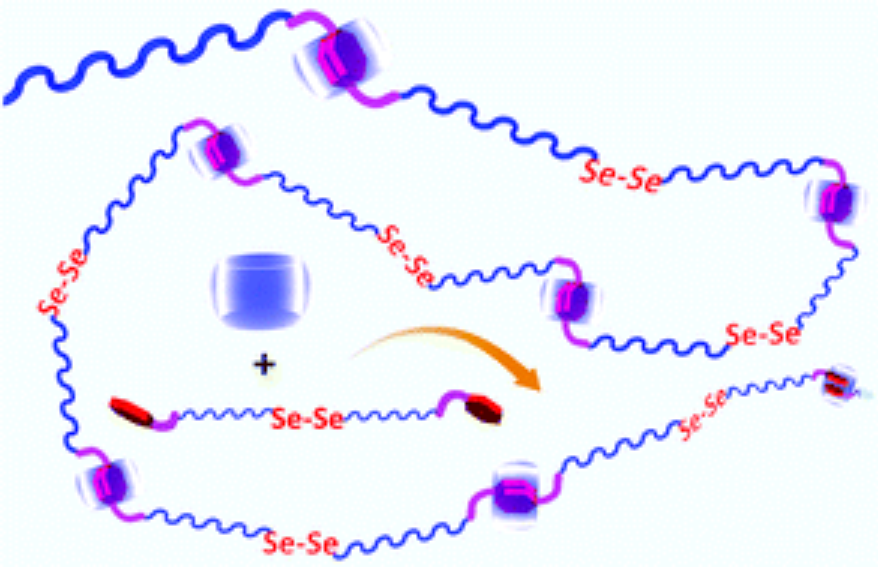
This communication describes the fabrication of diselenide-containing supramolecular polymers. The diselenide-containing supramolecular polymers were fabricated by equimolarly mixing (FGGC11Se)2 and CB[8] in aqueous solution driven by host–guest interactions between cucurbit[8]uril (CB[8]) and Phe-Gly-Gly. The amphiphilic diselenide-containing supramolecular polymers could further form aggregates for loading hydrophobic small molecules such as Nile Red and releasing them upon addition of oxidants or reductants. This line of research not only expands the library of selenium-containing polymers, but also offers a new strategy to develop functional supramolecular polymers for drug delivery.
28 Oct 2014, Journal of material chemistry A
Ru-Qiang Lu ,Yu-Qing Zheng, Yi-Nyu Zhou,Xiao-Yun Yan, Ting Lei, Ke Shi, Yan Zhou, Jian Pei, Laura Zoppi, Kim K. Baldridge, Jay S. Siegeld and Xiao-Yu Cao*
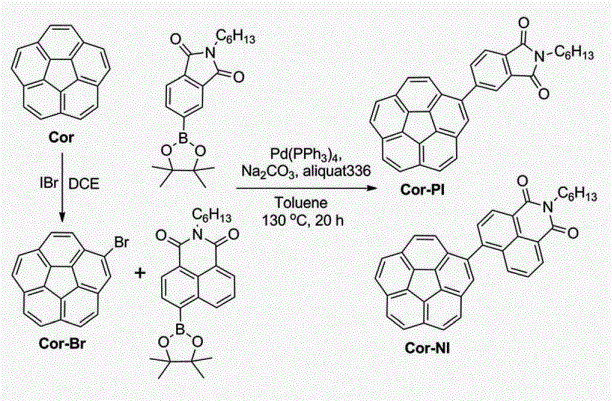
Corannulene derivatives were used in organic solar cells for the fifirst time. Using Cor-PI and Cor-NI as acceptors, we achieved power conversion effiffifficiencies up to 0.32% and 1.03%, suggesting potential applications of these fullerene segments as non-fullerene acceptors.28 October 2014, In Computer Graphics Forum
Shihui Guo, Jian Chang, Xiaosong Yang, Wencheng Wang, and Jianjun Zhang
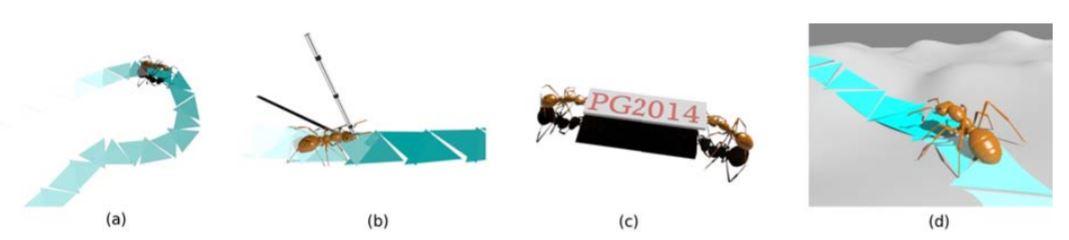
Natural‐looking insect animation is very difficult to simulate. The fast movement and small scale of insects often challenge the standard motion capture techniques. As for the manual key‐framing or physics‐driven methods, significant amounts of time and efforts are necessary due to the delicate structure of the insect, which prevents practical applications. In this paper, we address this challenge by presenting a two‐level control framework to efficiently automate the modeling and authoring of insects’ locomotion. On the top level, we design a Triangle Placement Engine to automatically determine the location and orientation of insects’ foot contacts, given the user‐defined trajectory and settings, including speed, load, path and terrain etc. On the low‐level, we relate the Central Pattern Generator to the triangle profiles with the assistance of a Controller Look‐Up Table to fast simulate the physically‐based movement of insects. With our approach, animators can directly author insects’ behavior among a wide range of locomotion repertoire, including walking along a specified path or on an uneven terrain, dynamically adjusting to external perturbations and collectively transporting prey back to the nest.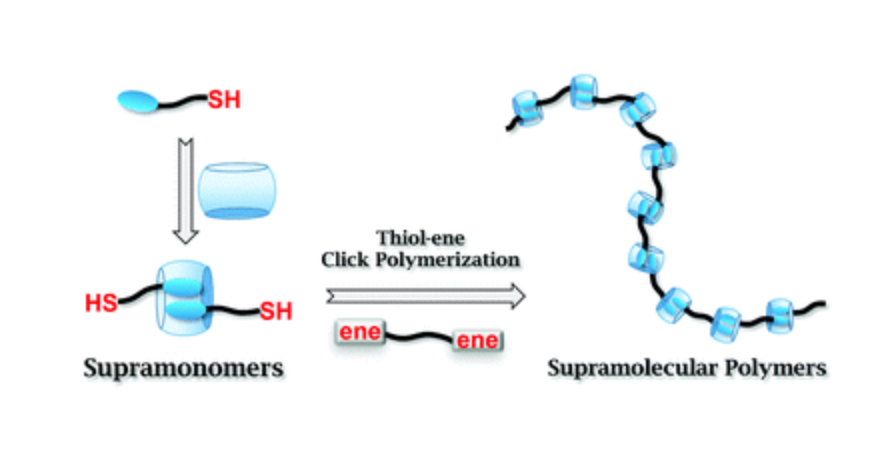
15 Oct 2014, Polymer Chemistry
Q. Song, F. Li, L. L. Yang, Z. Q. Wang, X. Zhang*
This communication describes the fabrication of supramonomers based on supramolecular complexation between cucurbit[8]uril and the tetrapeptide Phe-Gly-Gly-Cys. The supramolecular polymers are obtained by thiol–ene click polymerization of the supramonomers with maleimide-terminated poly(ethylene glycol). Benefiting from the green and pH dependent nature of thiol–ene click reaction, it is expected that this line of research will enrich the methodology for fabricating supramolecular polymers with controlled compositions and structures.10 Oct 2014, RSC Advanced
Ru-Qiang Lu, Wei Xuan, Yu-Qing Zheng, Yi-Nyu Zhou, Xiao-Yun Yan, Jin-Hu Dou, Rui Chen, Jian Pei, Wengui Weng and Xiao-Yu Cao
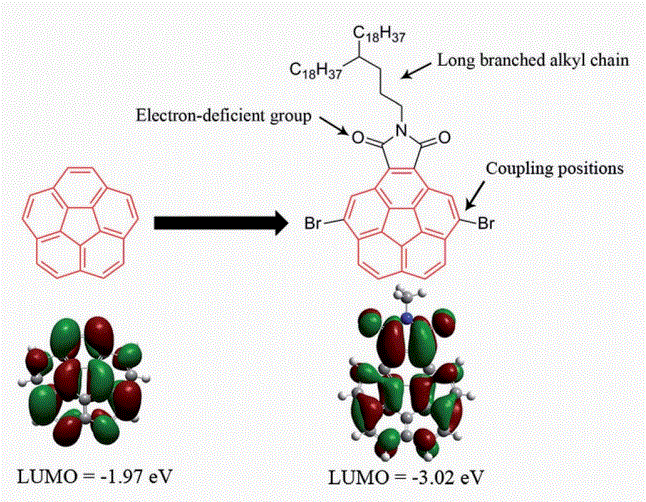
For the first time, the corannulene unit was incorporated directly into the backbone of conjugated polymers. A new donor–acceptor (D–A) copolymer PICBT using imide-fused corannulene as acceptor was synthesized and its performance in organic field-effect transistors (OFETs) was tested. PICBT exhibited ambipolar transporting property with a hole mobility of 0.025 cm2 V�1 s�1 and electron
mobility of 7.45 × 10-5 cm2V-1s-1 when the substrates were treated with octyltrimethoxysilane (OTS).If the substrates were not modified with OTS, PICBT showed lower device performances with a hole mobility of 4.62 × 10-3 cm2 V-1s-1 and electron mobility of 1.54 × 10-4 cm2 V-1 s-1. The device performances are competitive among the amorphous materials. This work paved the way for incorporating the corannulene unit into conjugated materials.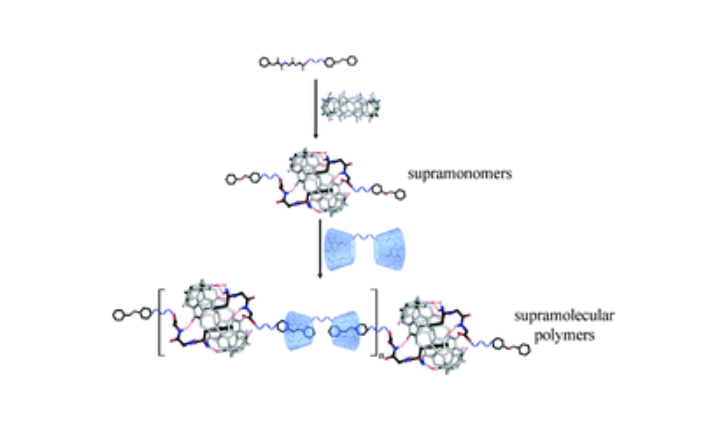
16 Jul 2014, Polymer Chemistry
Q. Song, F. Li, X. X. Tang, L. L. Yang, Z. Q. Wang, X. Zhang*
This communication describes a new method of fabricating supramolecular polymers through supramolecular polymerization of supramonomers. To mix building blocks of Phe-Gly-Gly linked with an azobenzene group and cucurbit[8]uril (CB[8]) in a molar ratio of 2 : 1 in aqueous solutions, supramonomers were obtained by host–guest interaction between tripeptide and CB[8]. Then supramolecular polymers were formed spontaneously by mixing the supramonomers with bis-β-cyclodextrins in a molar ratio of 1 : 1 in an aqueous solution through noncovalent host–guest complexation of the azobenzene group and β-cyclodextrin. Considering that various noncovalent interactions can be used to drive the formation of supramonomers and the supramolecular polymerization of the supramonomers, this study can enrich the methodology of fabricating supramolecular polymers.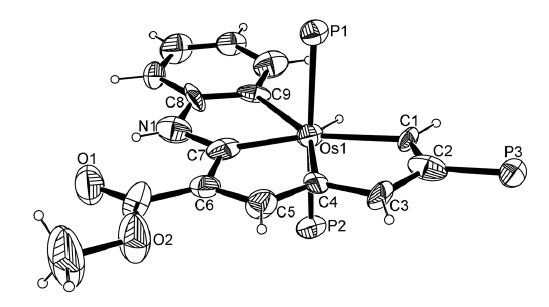
29 April 2014, Angewandte Chemie International Edition
Congqing Zhu, Qin Zhu, Jinglan Fan, Jun Zhu, Xumin He, Xiao-Yu Cao,* and Haiping Xia*
Aromaticity is one of the most important concepts in organic chemistry. A variety of metalla‐aromatic compounds have been recently prepared and in most of those examples, the metal participates only in a monocyclic ring. In contrast, metal‐bridged bicyclic aromatic molecules, in which a metal is shared between two aromatic rings, have been less developed. Herein, we report the first metal‐bridged tricyclic aromatic system, in which the metal center is shared by three aromatic five‐membered rings. These metalla‐aromatics are formed by reaction between osmapentalyne and arene nucleophiles. Experimental results and theoretical calculations reveal that the three five‐membered rings around the osmium center are aromatic. In addition, the broad absorption bands in the UV/Vis absorption spectra of these novel aromatic systems cover almost the entire visible region. This straightforward synthetic strategy may be extended to the synthesis of other metal‐bridged polycyclic aromatics.
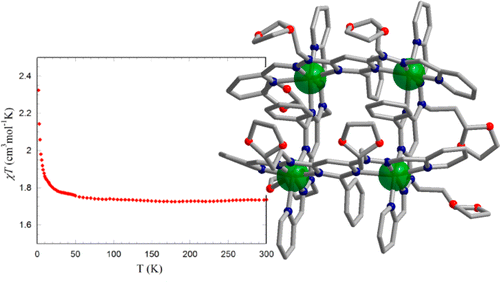
22 Apr 2014, Inorganic Chemistry
Augustin M. Madalan, Xiao-Yu Cao, Guillaume Rogez, and Jean-Marie Lehn*
Two copper(II) [2 × 2] grid-like complexes were synthesized and structurally characterized. Investigation of the magnetic properties showed for both the occurrence of intramolecular ferromagnetic interactions.
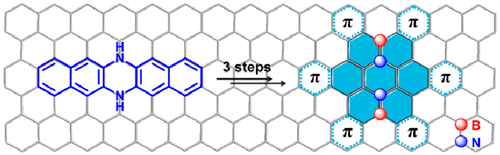
02 Mar 2014, Journal of American Chemistry Society
Xiao-Ye Wang, Fang-Dong Zhuang, Rui-Bo Wang, Xin-Chang Wang, Xiao-Yu Cao,*, Jie-Yu Wang,* and Jian Pei*
A straightforward strategy has been used to construct large BN-embedded π-systems simply from azaacenes. BN heterosuperbenzene derivatives, the largest BN heteroaromatics to date, have been synthesized in three steps. The molecules exhibit curved π-surfaces, showing two different conformations which are self-organized into a sandwich structure and further packed into a π-stacking column. The assembled microribbons exhibit good charge transport properties and photoconductivity, representing an important step toward the optoelectronic applications of BN-embedded aromatics.
Complex Assembly System © Copyright 2020