
2019
30 Jul 2019, Chemical Science
Yu Wang, Yibin Sun, Peichen Shi, Matthew M. Sartin, Xujing Lin, Pei Zhang, Hongxun Fang, Pixian Peng, Zhongqun Tian* and Xiaoyu Cao*
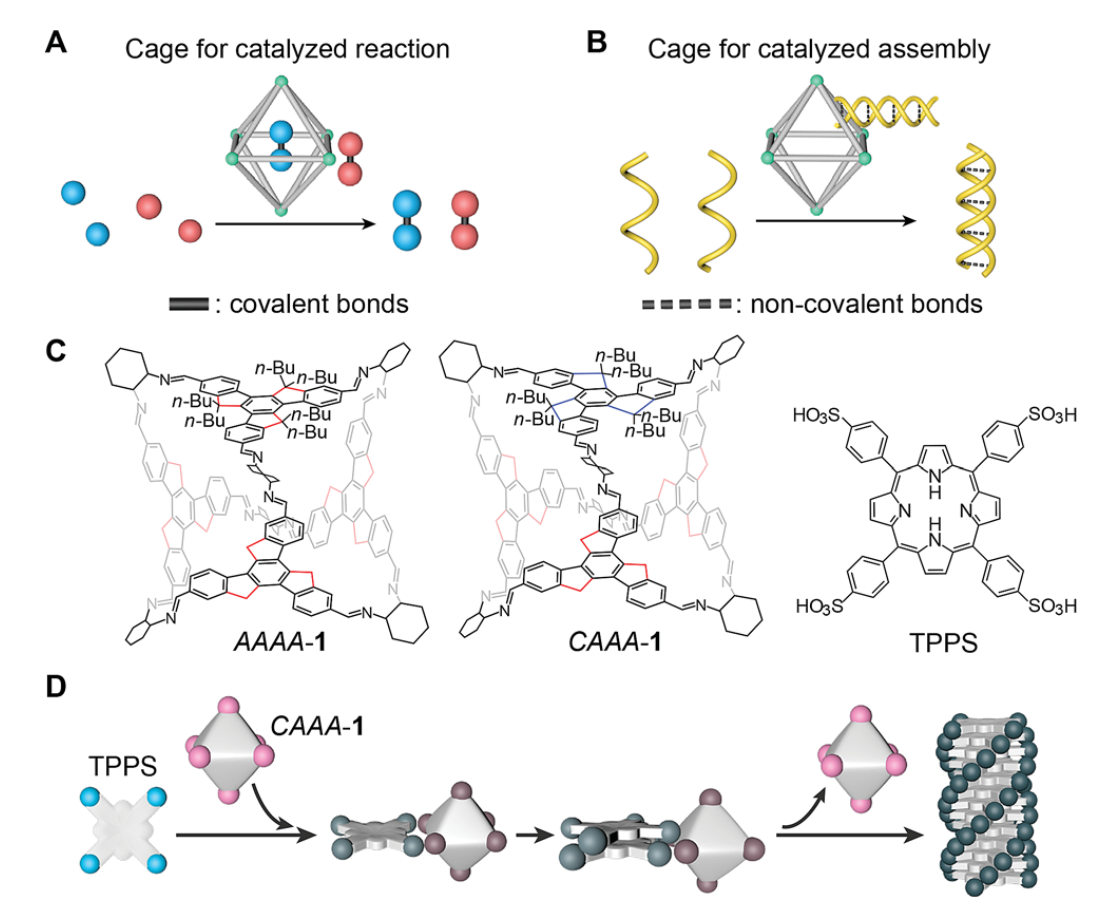
Cage catalysis has emerged as an important approach for mimicking enzymatic reactions by increasing the reaction rate and/or product selectivity of various types of covalent reactions. Here, we extend the catalytic application of cage compounds to the field of non-covalent molecular assembly. Acid-stable chiral imine cages are found to catalyze the supramolecular polymerization of porphyrins with an accelerated assembling rate and increased product enantioselectivity. Because the imine cages have a stronger interaction with porphyrin monomers and a weaker interaction with porphyrin assemblies, they can fully automatically detach from the assembled products without being consumed during the catalytic process. We reveal the kinetics of the auto-detachment of cages and the chirality growth of the assemblies using spectroscopic characterization studies. We find that the passivation groups attached to the cages are important for maintaining the structural stability of the cages during catalyzed assembly, and that the steric geometries of the cages can profoundly affect the efficiency of chiral regulation. This strategy demonstrates a new type of catalytic application of cage compounds in the field of molecular assembly, and paves the way to controlling supramolecular polymerization through a catalytic pathway.
07 Mar 2019, Angew. Chem. Int. Ed
Ru-Qiang Lu, Shuang Wu, Lin-Lin Yang, Wen-Bin Gao, Hang Qu, XiaoYe Wang, Jun-Bo Chen, Chun Tang, Hai-Yan Shi, and Xiao-Yu Cao*
The synthesis of open‐shell polycyclic hydrocarbons with large diradical characters is challenging because of their high reactivities. Herein, two diindeno‐fused corannulene regioisomers DIC‐1 and DIC‐2, curved fragments of fullerene C104, were synthesized that exhibit open‐shell singlet ground states. The incorporation of the curved and non‐alternant corannulene moiety within diradical systems leads to significant diradical characters as high as 0.98 for DIC‐1 and 0.89 for DIC‐2. Such high diradical characters can presumably be ascribed to the re‐aromatization of the corannulene π system. Although the DIC compounds have large diradical characters, they display excellent stability under ambient conditions. The half‐lives are 37 days for DIC‐1 and 6.6 days for DIC‐2 in solution. This work offers a new design strategy towards diradicaloids with large diradical characters yet maintain high stability.
Complex Assembly System © Copyright 2020

