
6 Mar 2024, Langmuir
Ding Zou, Xue Dong, Tianyi Tong, Wenbin Gao, Sheng He, Zhihao Li, Liulin Yang*, and Xiaoyu Cao*
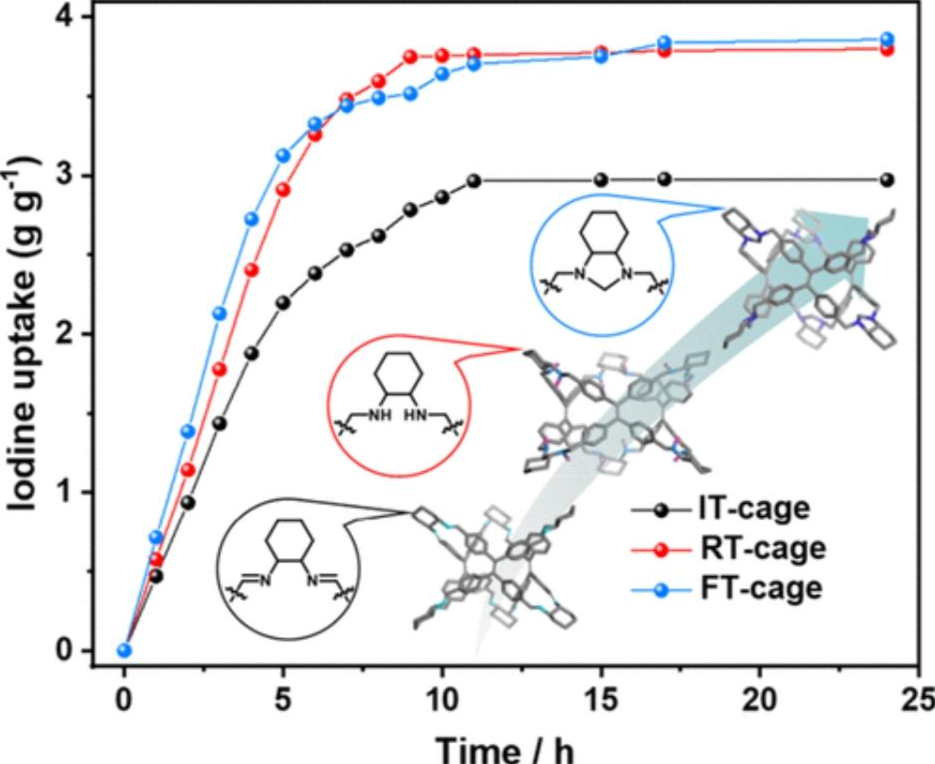
Iodine radioisotopes, produced or released during nuclear-related activities, severely affect human health and the environment. The efficient removal of radioiodine from both aqueous and vapor phases is crucial for the sustainable development of nuclear energy. In this study, we propose an “N-heteroatom engineering” strategy to design three porous organic cages with N-containing functional groups for efficient iodine capture. Among the molecular cages investigated, FT-Cage incorporating tertiary amine groups and RT-Cage with secondary amine groups show higher adsorption capacity and much faster iodine release compared to IT-Cage with imine groups. Detailed investigations demonstrate the superiority of amine groups, along with the influence of crystal structures and porosity, for iodine capture. These findings provide valuable insights for the design of porous organic cages with enhanced capabilities for capturing iodine.
19 Feb 2024, Accounts of Chemical Research
Xue Dong, Hang Qu, Andrew C.-H. Sue, Xin-Chang Wang*, and Xiao-Yu Cao*
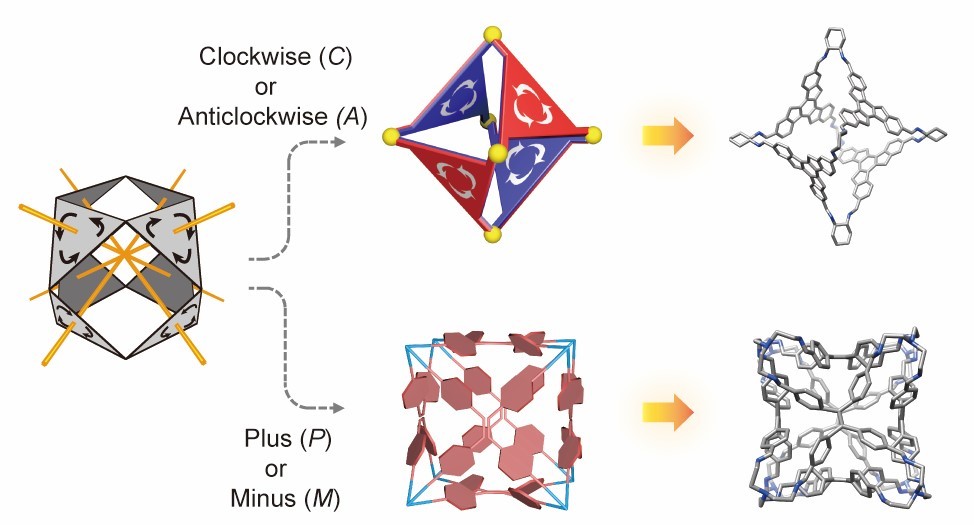
Molecular polyhedral cages, notable for their enclosed inner cavities, can possess varying degrees of symmetry, spanning from regular Platonic polyhedra to lower symmetry forms that may display chirality. Crafting chiral molecular cages typically involves using building blocks containing stereogenic elements or arranging achiral components in a manner that lacks mirror and inversion symmetries. Achieving precise control over their chirality poses both significance and challenges.
In this Account, we present an overview of our research endeavors in the realm of chiral molecular polyhedral cages, drawing inspiration from Buckminster Fuller’s “Face-Rotating Polyhedra (FRP)”. Mathematically, FRP introduce a unique form of chirality distinguished by a rotating pattern around the center of each face, setting it apart from regular polyhedra.
Molecular FRP can be constructed using two types of facial building blocks. The first includes rigid, planar molecules such as truxene and triazatruxene, which exhibit either clockwise or counterclockwise rotations in two dimensions. The second category involves propeller-like molecules, e.g., tetraphenylethylene, 1,2,3,4,5-penta(4-phenylaldehyde)pyrrole, and tridurylborane, displaying dynamic stereochemistry.
The synthesis of FRP may potentially yield a diverse array of stereoisomers. Achieving high stereoselectivity becomes feasible through the selection of building blocks with specific substitution patterns and rigidity. Prominent noncovalent repulsive forces within the resulting cages often play a pivotal role in the dynamic covalent assembly process, ultimately leading to the formation of thermodynamically stable FRP products.
The capacity to generate a multitude of stereoisomers, combined with the integration of chiral vertices, has facilitated investigations into phenomena such as chiral self-sorting and the “sergeant and soldiers” chiral amplification effect in FRP. Even the inclusion of one chiral vertex significantly impacts the stereochemical configuration of the entire cage. While many facial building blocks establish a stable rotational pattern in FRP, other units, such as tridurylborane, can dynamically transition between P and M configurations within the cage structures. The kinetic characteristics of such stereolabile FRP can be elucidated through physicochemical investigations.
Our research extends beyond the FRP concept to encompass mathematical analysis of these structures. Graph theory, particularly the coloring problem, sheds light on the intricate facial patterns exhibited by various FRP stereoisomers and serves as an efficient tool to facilitate the discovery of novel FRP structures. This approach offers a fresh paradigm for designing and analyzing chiral molecular polyhedral cages, showcasing in our work the synergy between mathematics and molecular design.
31 Jan 2024, Nature Materials
Xian-You Liu, Xiao-Yun Yan*, Yuchu Liu, Hang Qu, Yicong Wang, Jing Wang, Qing-Yun Guo, Huanyu Lei, Xing-Han Li, Fenggang Bian, Xiao-Yu Cao, Rui Zhang, Yu Wang, Mingjun Huang, Zhiwei Lin, E. W. Meijer, Takuzo Aida, Xian Kong* and Stephen Z. D. Cheng*
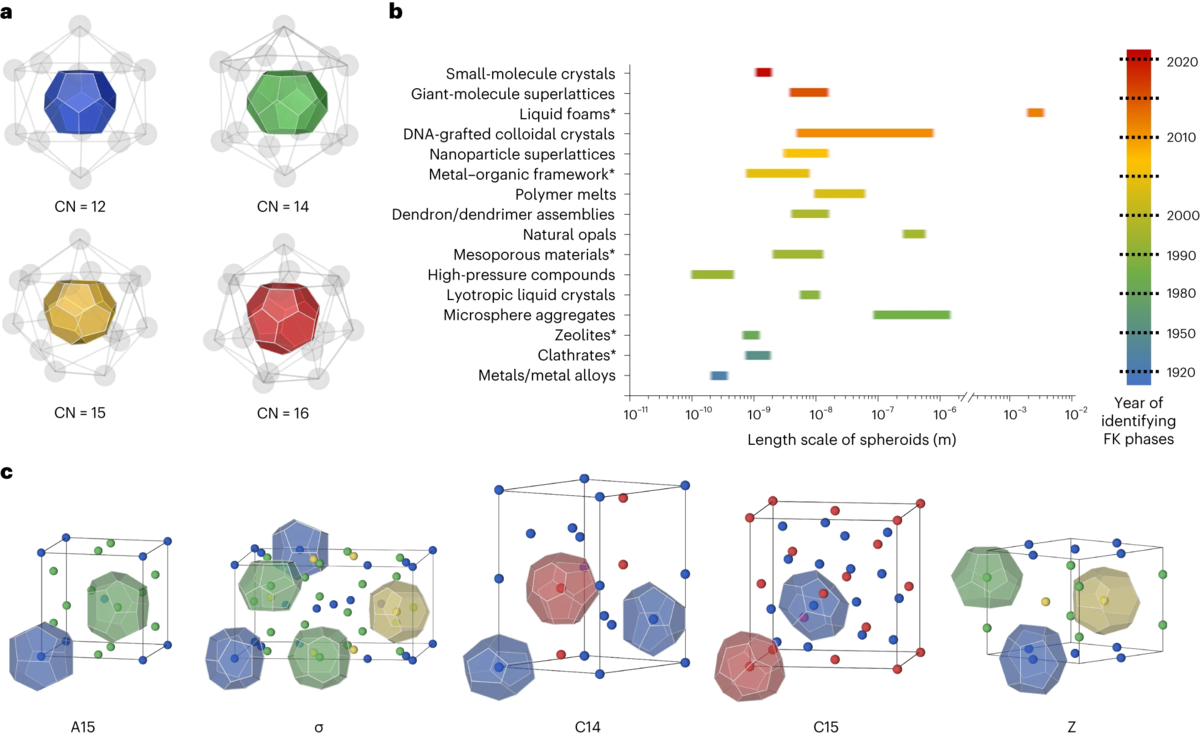
Soft building blocks, such as micelles, cells or soap bubbles, tend to adopt near-spherical geometry when densely packed together. As a result, their packing structures do not extend beyond those discovered in metallic glasses, quasicrystals and crystals. Here we report the emergence of two Frank–Kasper phases from the self-assembly of five-fold symmetric molecular pentagons. The μ phase, an important intermediate in superalloys, is indexed in soft matter, whereas the ϕ phase exhibits a structure distinct from known Frank–Kasper phases in metallic systems. We find a broad size and shape distribution of self-assembled mesoatoms formed by molecular pentagons while approaching equilibrium that contribute to the unique packing structures. This work provides insight into the manipulation of soft building blocks that deviate from the typical spherical geometry and opens avenues for the fabrication of ‘soft alloy’ structures that were previously unattainable in metal alloys.
17 Jan 2024, Chemical Society Reviews
Zhi-Chao Lei, Xinchang Wang, Liulin Yang*, Hang Qu, Yibin Sun, Yang Yang, Wei Li, Wen-Bin Zhang, Xiao-Yu Cao, Chunhai Fan, Guohong Li, Jiarui Wu and Zhong-Qun Tian*
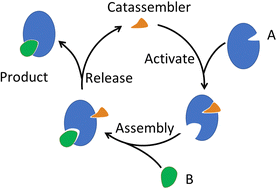
Molecular assembly is the process of organizing individual molecules into larger structures and complex systems. The self-assembly approach is predominantly utilized in creating artificial molecular assemblies, and was believed to be the primary mode of molecular assembly in living organisms as well. However, it has been shown that the assembly of many biological complexes is “catalysed” by other molecules, rather than relying solely on self-assembly. In this review, we summarize these catalysed-assembly (catassembly) phenomena in living organisms and systematically analyse their mechanisms. We then expand on these phenomena and discuss related concepts, including catalysed-disassembly and catalysed-reassembly. Catassembly proves to be an efficient and highly selective strategy for synergistically controlling and manipulating various noncovalent interactions, especially in hierarchical molecular assemblies. Overreliance on self-assembly may, to some extent, hinder the advancement of artificial molecular assembly with powerful features. Furthermore, inspired by the biological catassembly phenomena, we propose guidelines for designing artificial catassembly systems and developing characterization and theoretical methods, and review pioneering works along this new direction. Overall, this approach may broaden and deepen our understanding of molecular assembly, enabling the construction and control of intelligent assembly systems with advanced functionality.
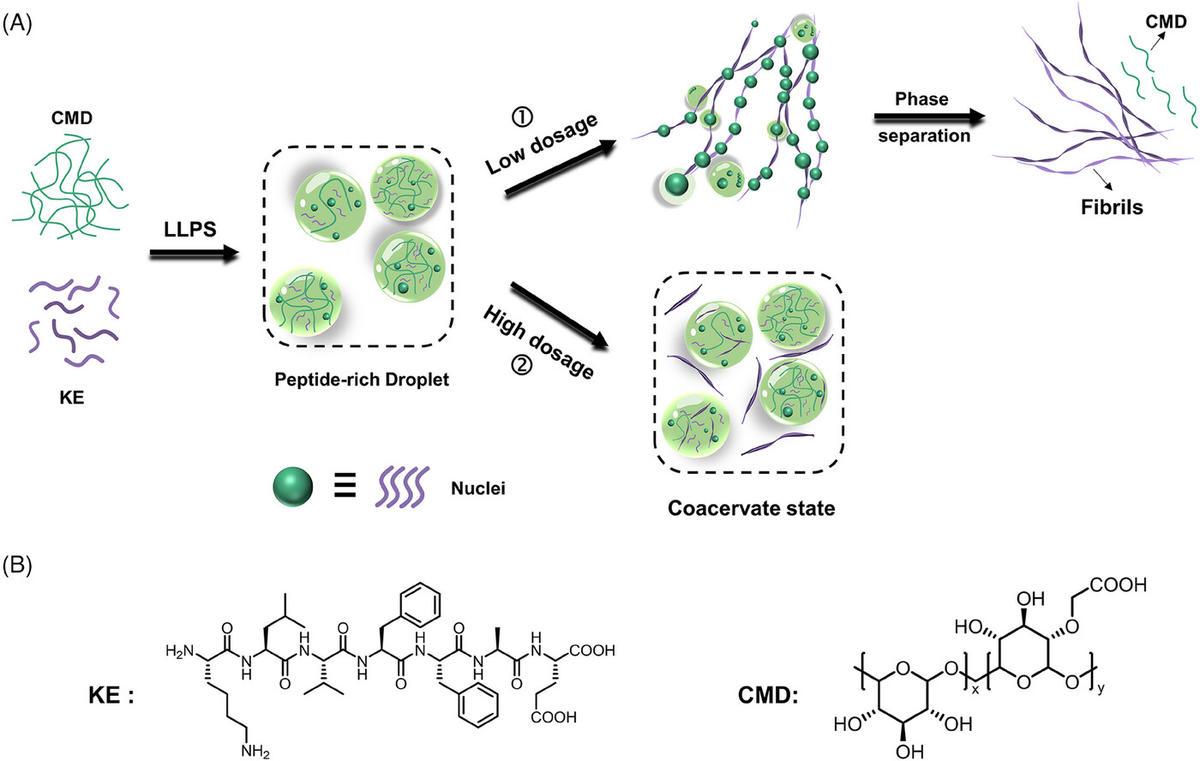
04 Jan 2024, Aggregate
Wang Li, Yang Zhou, Sheng He, Tianyi Tong, Congsen Wang, Peichen Shi, Suixu Li, Xinchang Wang, Liulin Yang*, Xiaoyu Cao* and Zhong-Qun Tian
In biological systems, molecular assembly primarily relies on the assistance ofmolecular chaperones. Inspired by nature, strategies like ‘chaperone-assisted assem-bly’ and ‘catalyzed assembly’ have been proposed for the sophisticated controlof molecular assembly. Nonetheless, significant challenges remain in the rationaldesign of such systems, calling for a deep understanding of underlying principles.Herein, we demonstrate an artificial chaperone serves a dual role, that is catalystin low dosages and inhibitor in high dosages, in regulating the supramolecularpolymerization of peptides. Low dosages of carboxymethyl cellulose, as the chaper-ones, catalyze the assembly of Aβ16-22peptides into fibrils through multi-step phaseseparation, while high dosages trap the peptides into coacervate intermediates andtherefore inhibit the fibrillation. Consequently, the quantity of chaperones does notfollow the intuition that ‘more is better’ for catalyzing assembly but instead has anoptimal molar ratio. Investigation reveals that the interplay and evolution of electro-static and hydrophobic interactions are the keys to achieving these processes. Thisstudy provides insights into the multifaceted roles artificial chaperones may play ina dosage-dependent manner and enriches the toolkit for efficient and controllableconstruction of complex assembly syste.
Complex Assembly System © Copyright 2020




