
2016
14 Dec 2016, Angewandte Chemie International Edition
Yunbin Hu, Xiao-Ye Wang, Pi-Xian Peng, Xin-Chang Wang, Xiao-Yu Cao, Xinliang Feng, Klaus Mgllen,* and Akimitsu Narita*
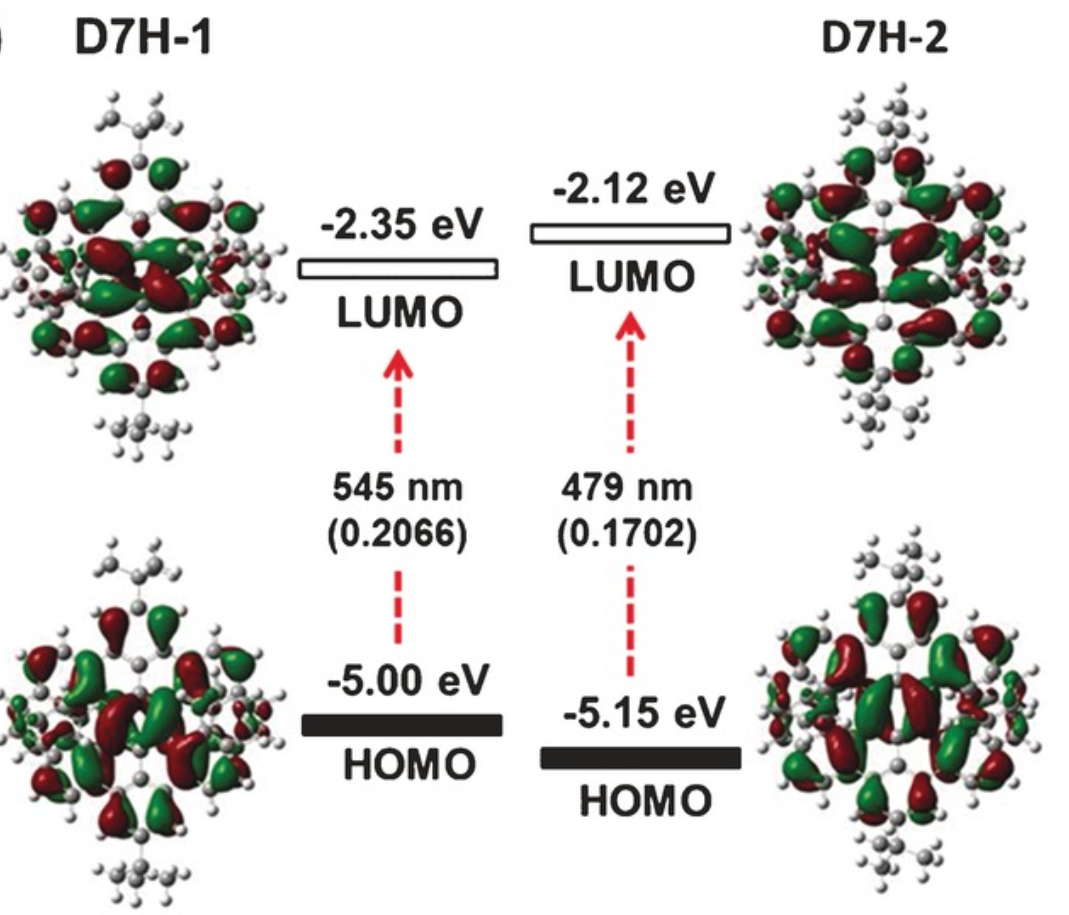
A benzo‐fused double [7]carbohelicene (D7H) was synthesized through a regioselective cyclodehydrogenation of a tetranaphthyl‐p‐terphenyl‐based precursor. The twisted (D7H‐1) and anti‐folded (D7H‐2) conformers of D7H were separated by recrystallization, and their double helicene structures with overlapping terminal benzene rings were unambiguously elucidated by X‐ray crystallography. A record‐high isomerization barrier (46.0 kcal mol−1) in double helicenes was estimated based on density functional theory (DFT) calculation, which resulted in the excellent conformational stability of D7H. The physicochemical properties of D7H‐1 and D7H‐2 were investigated by UV/Vis absorption spectroscopy and cyclic voltammetry, displaying the variation of electronic structure upon conformational changes. The optical resolution of the racemic D7H‐1 was carried out by chiral HPLC, offering enantiopure D7H‐1‐(P,P) and D7H‐1‐(M,M), which were further characterized by circular dichroism spectroscopy.
23 Nov 2016, Journal of American Chemistry Society
R. C. Fang, H. C. Zhang, L. L. Yang, H. T. Wang, Y. Tian*, X. Zhang*, L. Jiang*
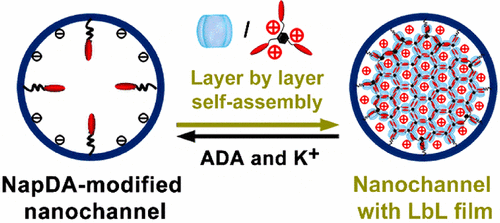
Artificial nanochannels, inheriting smart gating functions of biological ion channels, promote the development of artificial functional nanofluidic devices for high-performance biosensing and electricity generation. However, gating states of the artificial nanochannels have been mainly realized through chemical modification of the channels with responsive molecules, and their gating states cannot be further regulated once the nanochannel is modified. In this work, we employed a new supramolecular layer-by-layer (LbL) self-assembly method to achieve reversible and adjustable multiple gating features in nanofluidic diodes. Initially, a self-assembly precursor was modified into a single conical nanochannel, then host molecule-cucurbit[8]uril (CB[8]) and guest molecule, a naphthalene derivative, were self-assembled onto the precursor through an LbL method driven by host-enhanced π–π interaction, forming supramolecular monolayer or multilayers on the inner surface of the channel. These self-assemblies with different layer numbers possessed remarkable charge effects and steric effects, exhibiting a capability to regulate the surface charge density and polarity, the effective diameter, and the geometric asymmetry of the single nanochannel, realizing reversible gating of the single nanochannel among multiple rectification and ion-conduction states. As an example of self-assembly of supramolecular networks in nanoconfinements, this work provides a new approach for enhancing functionalities of artificial nanochannels by LbL supramolecular self-assemblies. Meanwhile, since the host molecule, CB[8], used in this work can interact with different kinds of biomolecules and stimuli-responsive chemical species, this work can be further extended to build a novel stable multiple-state research platform for a variety of uses such as sensing and controllable release.
22 Sep 2016, Journal of American Chemistry Society
Xiao-Ye Wang, Xin-Chang Wang, Akimitsu Narita,* Manfred Wagner, Xiao-Yu Cao,* Xinliang Feng, and Klaus Müllen*
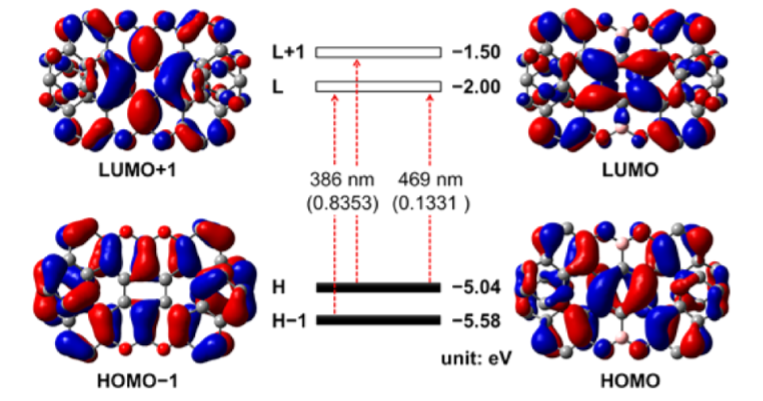
The synthesis of 11a,25a-dibora-11,12,25,26-tetraoxatetranaphtho[1,2-a:2′,1′-f:1″,2″-j:2‴,1‴-o]perylene, a double [7]heterohelicene containing OBO units, has been achieved via tandem demethylation-borylation, representing the highest double helicene reported thus far with all six-membered rings. Single-crystal X-ray analysis clearly demonstrated a significantly twisted structure with the terminal aromatic rings overlapping at both ends, giving the first example of a double helicene with intramolecular π-layers. Such structural features resulted in a high theoretical isomerization barrier of 45.1 kcal/mol, which is the highest value for all the double helicenes ever reported, rendering the achieved molecule with high chiral stability. The (P,P)- and (M,M)-isomers were separated by chiral HPLC and the chiroptical properties were investigated, revealing opposite circular dichroism responses.
24 Aug 2016, Nature Communications
XinChang Wang, Yu Wang, Huayan Yang, Hongxun Fang, Ruixue Chen, Yibin Sun, Nanfeng Zheng, Kai Tan, Xin Lu, Zhongqun Tian* and Xiao-Yu Cao*
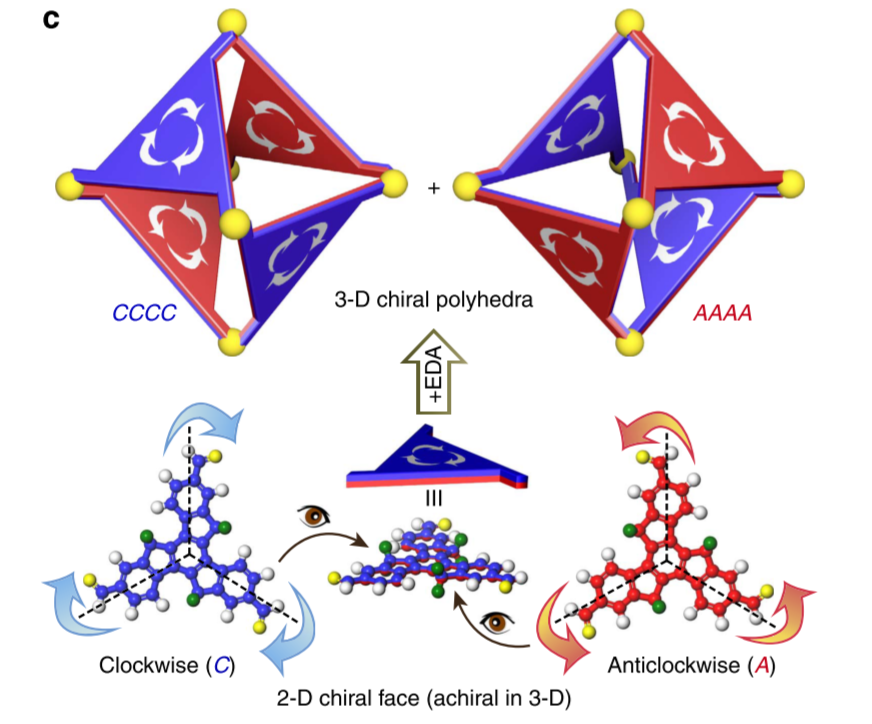
In nature, protein subunits on the capsids of many icosahedral viruses form rotational patterns, and mathematicians also incorporate asymmetric patterns into faces of polyhedra. Chemists have constructed molecular polyhedra with vacant or highly symmetric faces, but very little is known about constructing polyhedra with asymmetric faces. Here we report a strategy to embellish a C3h truxene unit with rotational patterns into the faces of an octahedron, forming chiral octahedra that exhibit the largest molar ellipticity ever reported, to the best of our knowledge. The directionalities of the facial rotations can be controlled by vertices to achieve identical rotational directionality on each face, resembling the homo-directionality of virus capsids. Investigations of the kinetics and mechanism reveal that non-covalent interaction among the faces is essential to the facial homo-directionality.
Complex Assembly System © Copyright 2020



