
1 Sep 2023, Journal of Materials Chemistry B
Yuqing Su#, Beibei Liu#, Zhenkun Huang#, Zihao Teng, Liulin Yang, Jie Zhu, Shuaidong Huo* and Aijie Liu*
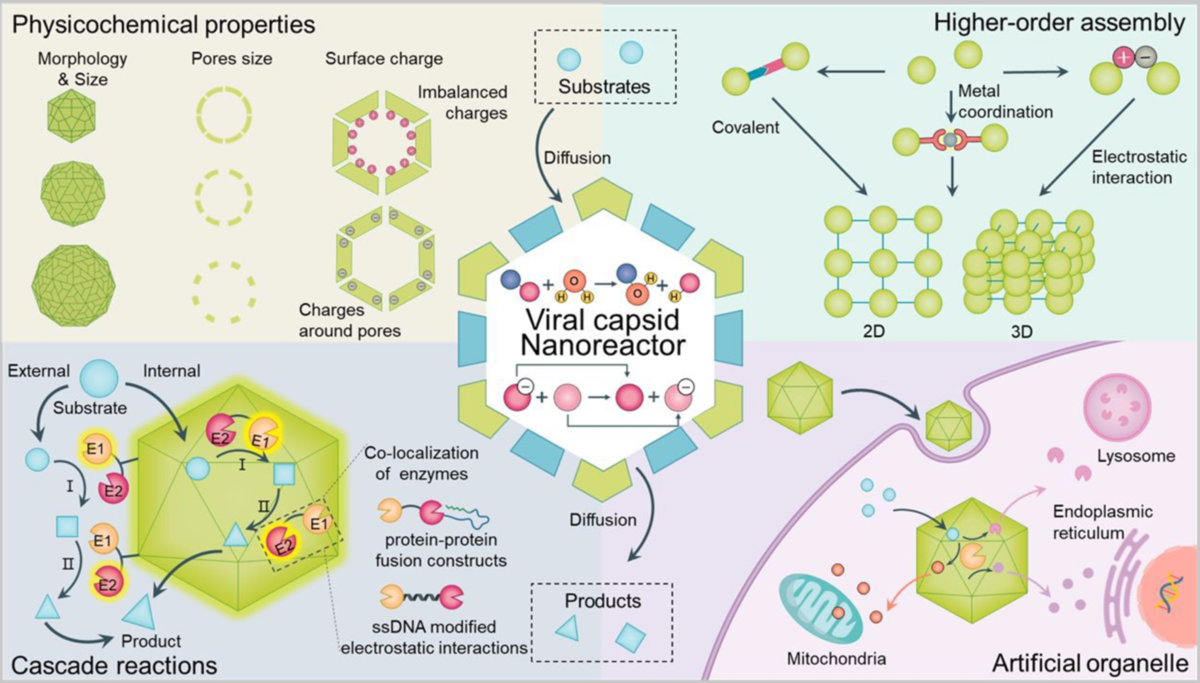
Viral protein cages are self-assembled supramolecular structures that can be found in nature for compartmentalization. Exploiting their inherent properties, including precise nanoscale structures, monodispersity, and high stability, these architectures have been widely used as nanocarriers to protect or enrich catalysts, facilitating catalytic reactions and avoid interference from the bulk solutions. In this review, we summarize the current progress of viral protein cage-based nanoreactors. First, we briefly introduce the physiochemical properties of the most commonly used viral protein cages to understand their roles in catalytic reactions beyond the confined space. Next, we summarize the self-assembly of nanoreactors forming higher-order hierarchical structures, highlighting the emerging field of protein cages as artificial organelles and their potential biomedical applications. Finally, we discuss the current findings and future perspectives of protein cage-based nanoreactors.
1 Aug 2023, Journal of American Chemistry Society
Wenbin Gao, Zhihao Li, Tianyi Tong, Xue Dong, Hang Qu*, Liulin Yang, Andrew C.-H. Sue*, Zhongqun Tian, and Xiaoyu Cao*
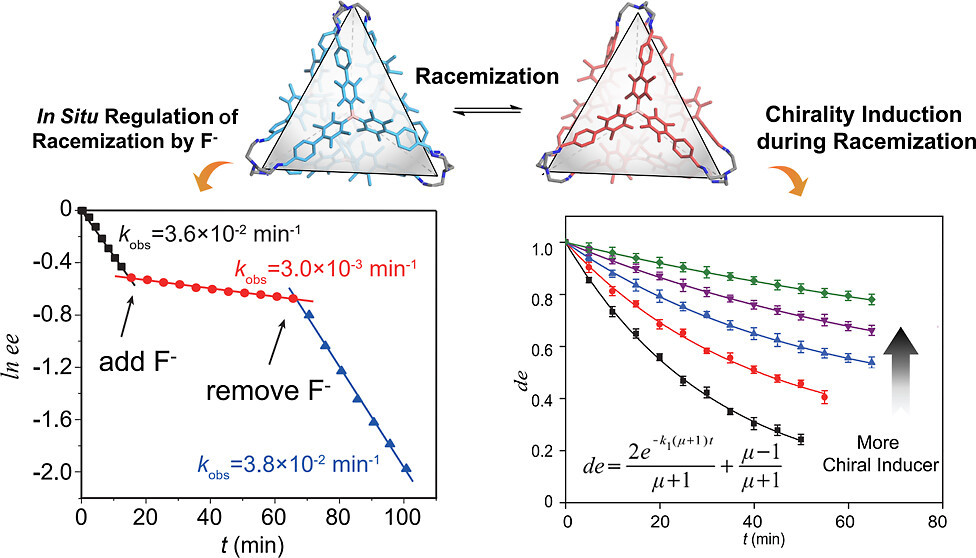
The manipulation of chirality in molecular entities that rapidly interconvert between enantiomeric forms is challenging, particularly at the supramolecular level. Advances in controlling such dynamic stereochemical systems offer opportunities to understand chiral symmetry breaking and homochirality. Herein, we report the synthesis of a face-rotating tetrahedron (FRT), an organic molecular cage composed of tridurylborane facial units that undergo stereomutations between enantiomeric trefoil propeller-like conformations. After resolution, we show that the racemization barrier of the enantiopure FRT can be regulated in situ through the reversible binding of fluoride anions onto the tridurylborane moieties. Furthermore, the addition of an enantiopure phenylethanol to the FRT can effectively induce chirality of the molecular cage by preferentially binding to one of its enantiomeric conformers. This study presents a new paradigm for controlling dynamic chirality in supramolecular systems, which may have implications for asymmetric synthesis and dynamic stereochemistry.
6 Jun 2023, Journal of Materials Chemistry B
Shuqin Cao, Sandro Peeters, Sandra Michel-Souzy, Sandra Michel-Souzy, Sandra Michel-Souzy, Liulin Yang*, and Jeroen J. L. M. Cornelissen*
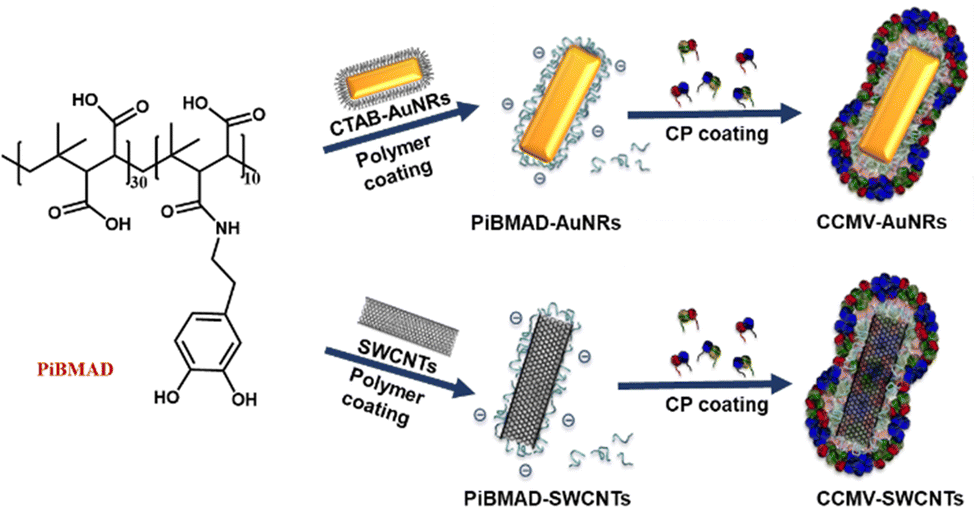
A generic strategy to construct virus protein-based hybrid nanomaterials is reported by using a macromolecular glue inspired by mussel adhesion. Commercially available poly(isobutylene-alt-maleic anhydride) (PiBMA) modified with dopamine (PiBMAD) is designed as this macromolecular glue, which serves as a universal adhesive material for the construction of multicomponent hybrid nanomaterials. As a proof of concept, gold nanorods (AuNRs) and single-walled carbon nanotubes (SWCNTs) are initially coated with PiBMAD. Subsequently, viral capsid proteins from the Cowpea Chlorotic Mottle Virus (CCMV) assemble around the nano-objects templated by the negative charges of the glue. With virtually unchanged properties of the rods and tubes, the hybrid materials might show improved biocompatibility and can be used in future studies toward cell uptake and delivery.
3 Mar 2023, ACS Appl. Mater. Interfaces
Ding Zou#, Zhihao Li#, Da Long, Xue Dong, Hang Qu, Liulin Yang*, and Xiaoyu Cao*
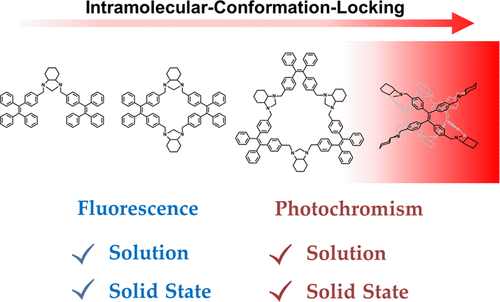
The rational design of stimuli-responsive materials requires a deep understanding of the structure–activity relationship. Herein, we proposed an intramolecular conformation-locking strategy─incorporating flexible tetraphenylethylene (TPE) luminogens into the rigid scaffold of a molecular cage─to produce a molecular photoswitch with dual outputs of luminescence and photochromism in solution and in the solid states at once. The molecular cage scaffold, which restricts the intramolecular rotations of the TPE moiety, not only helps to preserve the luminescence of TPE in a dilute solution but facilitates the reversible photochromism on account of the intramolecular cyclization/cycloreversion reactions. Furthermore, we demonstrate assorted applications of this multiresponsive molecular cage, e.g., photo-switchable patterning, anticounterfeiting, and selective vapochromism sensing.
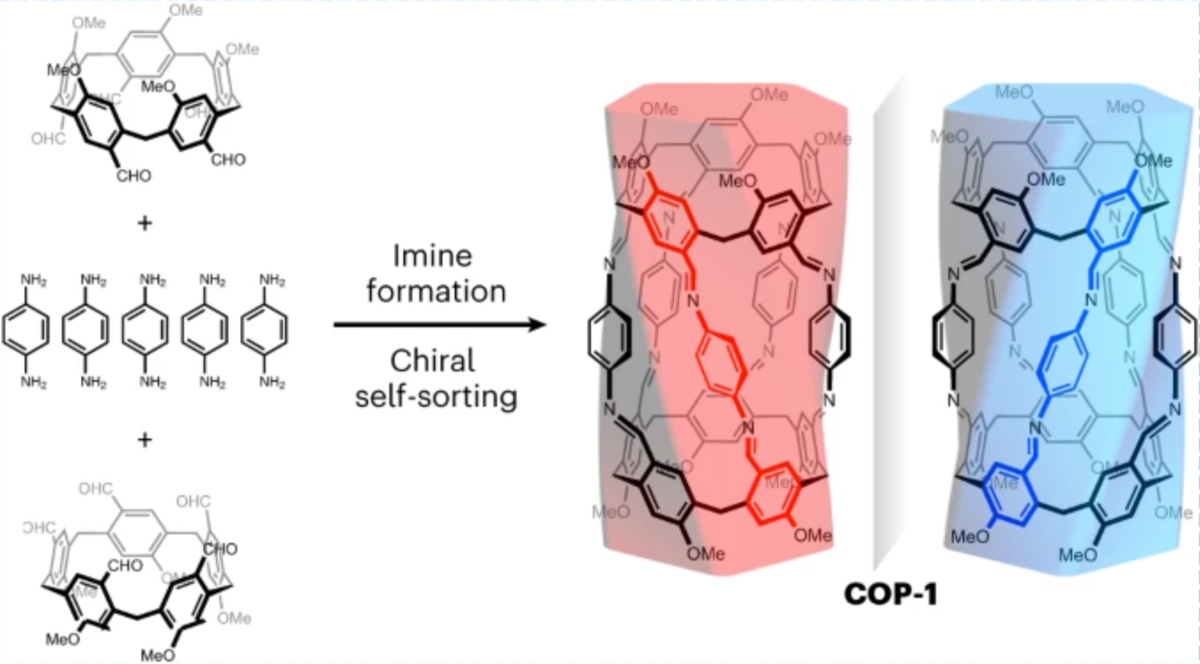
13 Feb 2023, Nature Synthesis
Yaru Tian, Yunlong Guo, Xue Dong, Xintong Wan, Kuan-Heng Cheng, Rong Chang, Shunshun Li, Xiaoyu Cao, Yi-Tsu Chan & Andrew C.-H. Sue*
The construction of nanotubes with well-defined structures, although synthetically challenging, offers the prospect of studying novel chemical reactions and transportation within confined spaces, as well as fabricating molecular devices and nanoporous materials. Here we report a discrete molecular nanotube, namely the covalent organic pillar COP-1, synthesized through a [2 + 5] imine condensation reaction involving two penta-aldehyde macrocycles and five phenylenediamine linkers. A pair of enantiomeric nanotubes, obtained in a quantitative and diastereoselective manner, were characterized and resolved readily. NMR spectroscopy, isothermal titration calorimetric and X-ray crystallographic studies revealed that the 2-nm-long and 4.7-Å-wide one-dimensional channel inside COP-1 can accommodate α,ω-disubstituted n-alkyl chains with complementary lengths and electron density distributions. Furthermore, in a length-mismatched host–guest pair, we found that the nonamethylene dibromide thread not only displays a diminished binding constant in solution, but adapts an energetically unfavoured gauche conformation inside COP-1 in the solid state.
Complex Assembly System © Copyright 2020




